2. 济宁医学院免疫学研究所, 济宁 272067
2. Institute of Immunology and Molecular Medicine, Jining Medical University, Jining 272067, China
在全球范围内,乳腺癌是女性最常见的恶性肿瘤之一,也是女性癌症死亡的主要原因[1-2]。乳腺癌有3个重要的生物标志物:雌激素受体(estrogen receptor,ER),孕激素受体(progesterone receptor,PR)和人表皮生长因子受体(human epidermal growth factor receptor,HER2/neu)。根据ER、PR、HER2的表达和肿瘤细胞增殖指标ki-67,乳腺癌可分为4种类型:Luminal A,Luminal B,HER2(+)和三阴性乳腺癌。目前临床上主要根据乳腺癌的类型制定治疗方案主要包括手术、化疗、放疗、内分泌治疗及靶向治疗[3]。但是,肿瘤微环境中复杂的免疫环境往往导致常规治疗方案的效果大打折扣。
髓源性抑制细胞(myeloid-derived suppressor cells, MDSCs)是一种异质性骨髓祖细胞群,包括未成熟的粒细胞,巨噬细胞(macrophage)和树突状细胞(dendritic cells, DC)[4]。在小鼠中,MDSCs多表达Gr1和CD11b。在人类中,MDSCs主要存在于血液和各种器官的肿瘤微环境中,以Lin-/loCD33+CD11b+HLA-DR-为表征。研究发现乳腺癌细胞可以募集包括MDSCs、调节性T细胞(regulatory T cells, Treg)和Ⅱ型巨噬细胞在内的肿瘤浸润性淋巴细胞来构成促肿瘤微环境,进而抑制抗肿瘤免疫反应[5]。此外,MDSCs诱导的免疫耐受可促进乳腺癌的进展和转移。血液中MDSCs水平与乳腺癌分期及预后相关,较高水平的MDSCs可能增加乳腺癌的复发率和转移率[6]。本文将MDSCs在乳腺癌中的生物学功能及调控机制作一综述,重点介绍乳腺癌MDSCs的募集及其机制、MDSCs对乳腺癌的作用以及以MDSCs为靶向的免疫治疗。
1 MDSCs在乳腺癌中的募集及机制乳腺癌细胞可以产生调节MDSCs的细胞因子和趋化因子,通过肿瘤源性的细胞因子和趋化因子将MDSCs从骨髓募集到肿瘤部位。在乳腺癌中,与MDSCs分化有关的细胞因子包括粒细胞集落刺激因子(granulocyte colony stimulating factor, G-CSF)、粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony stimulating factor, GM-CSF)、白介素6(interleukin-6, IL-6)、白介素1(interleukin-1β, IL-1β)、巨噬细胞迁移抑制因子(macrophage migration inhibitory factor, MIF)和转化生长因子(transforming growth factor-β1, TGF-β1)。研究表明,接种4T1细胞的BALB/c小鼠中,G-CSF、GM-CSF、IL-6、MIF和TGF-β1均能促进其骨髓细胞分化成MDSCs[7]。虽然以上细胞因子都与乳腺癌中MDSCs的募集和增殖有关,但每种细胞因子及各种细胞因子之间的相互作用对MDSCs的作用机制尚未明确。参与乳腺癌MDSCs迁移的趋化因子包括趋化因子生长调节基因5(C-X-C motif ligand 5, CXCL5),趋化因子1(C-C motif ligand 1, CCL1)和趋化因子5(C-C motif ligand 5, CCL5)[8]。研究发现,CXCL5与受体(C-X-C motif receptor 2, CXCR2)能够相互作用,对乳腺癌小鼠模型中的MDSCs募集起关键作用[9]。CCL1和CCL2也可以促进4T1乳腺癌小鼠模型中MDSCs的募集[10]。Kruppel样转录因子(krüppel-like factor4/KLF4, KLF4)可通过CXCL5诱导GM-CSF产生,从而维持乳腺癌MDSCs的数量[11]。除乳腺癌细胞衍生因子外,还有其他因素参与MDSCs的募集。一项随机临床试验表明,心理应激可通过上调G-CSF和IL-6来调节乳腺癌细胞MDSCs的水平[12]。此外,在乳腺癌患者的肺和肝脏中,高表达的S100钙结合蛋白家族8(S100 calcium binding protein A8, S100A8)可将MDSCs募集到这些靶器官,这也是促进乳腺癌转移的一种机制[13]。
2 MDSCs在乳腺癌中的作用及分子机制 2.1 MDSCs与乳腺癌免疫逃逸机制 2.1.1 STAT3/NF-κB/IDO途径吲哚胺2, 3-双加氧酶(indoleamine 2, 3-dioxygenase, IDO)是一种在癌症免疫治疗中具有关键作用的胞内酶,它可以使肿瘤细胞逃避抗肿瘤免疫。IDO可通过消耗色氨酸产生色氨酸代谢物来抑制效应T细胞,并可通过激活Treg细胞来影响全身免疫耐受。乳腺癌细胞分泌的IL-6可激活小鼠MDSCs中的信号传导及转录激活因子3 (signal transducers and activators of transcription3, STAT3),STAT3激活核因子(Nuclear factor-κB, NF-κB)亚基p52并导致亚基RelB易位至细胞核与IDO启动子序列直接结合,促进IDO的表达。Wei等[14]研究表明,IDO除了影响机体免疫耐受之外,还可以通过刺激肿瘤血管生成促进肿瘤转移。见图 1。
2.1.2 STAT3/IRF-8途径干扰素调节因子-8 (interferon regulatory factor-8, IRF-8)是人MDSCs的负调节因子。IRF-8的下调伴随着MDSCs数量的增加。接种4T1细胞的BALB/c小鼠中G-CSF和GM-CSF可经由MDSCs中的STAT3和STAT5途径下调IRF-8的转录水平[6]。Meyer等[15]已经证明乳腺癌细胞通过调节IRF8表达改变树突状细胞(dendritic cells, DC)与MDSCs的平衡,该过程有利于形成促进肿瘤转移的免疫环境。以上结果表明肿瘤可以通过调节炎症细胞因子,如G-CSF,改变转录因子IRF-8的表达来提高MDSCs的比例和功能,从而减弱机体的免疫防御。见图 1。
2.1.3 PTEN/Akt途径细胞外基质(extracellular matrix, ECM)是抵抗细胞侵袭的第一道屏障,而ECM蛋白的降解是肿瘤转移的重要步骤。研究发现,TGF-β1可诱导乳腺癌小鼠MDSCs miRNA-494上调,进而靶向下调磷酸酯酶与张力蛋白同源物(phosphatase and tensin homolog, PTEN)的表达。PTEN的下调导致Akt通路的激活(包括mTOR和NF-κB通路),并最终增加基质金属蛋白酶(matrix metalloproteinases, MMPs), 包括MMP2,MMP13和MMP14的表达,从而促进乳腺癌的浸润和转移。MMPs属于锌和钙依赖性细胞外酶家族,共有5个亚组(胶原酶、明胶酶、溶基质素、基质溶素和膜金属蛋白酶)。因此,MMPs可能在乳腺原位癌向侵袭性乳腺癌的转化中起关键作用[16]。见图 1。
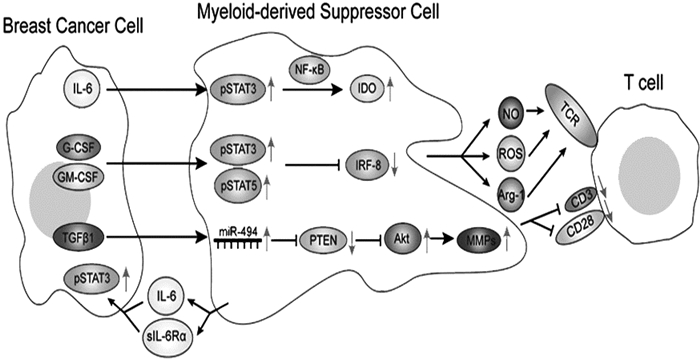
|
图 1 MDSCs在乳腺癌中发挥作用的分子机制及MDSCs在乳腺癌中的促肿瘤作用及机制[16] |
在乳腺癌微环境中,MDSCs以多种方式抑制T细胞,包括精氨酸酶(arginase1, ARG1)、活性氧(reactive oxygen species, ROS)、活性氮(reactive nitrogen species, RNS)和一氧化氮(nitric oxide, NO)等途径。MDSCs可通过ROS和RNS产生自由基,例如过氧亚硝基(Peroxynitrite, PNT)。PNT导致T细胞表面受体(T cell receptor, TCR)和白细胞分化抗原8(cluster of differentiation 8, CD8)分子的硝基化/亚硝基化,从而诱导T细胞免疫耐受。MDSCs也可以通过下调CD3/CD28的表达,进而抑制T细胞增殖[17]。
2.2.2 MDSCs对乳腺癌细胞的作用MDSCs被募集到乳腺癌微环境后,可表达IL-6和可溶性IL-6Rα,再通过IL-6反式信号转导促进乳腺癌细胞中STAT3磷酸化,促进乳腺癌的侵袭和转移。此外,骨溶解是乳腺癌的一个并发症,与MDSCs有关。MDSCs通过一氧化氮信号通路与乳腺癌细胞相互作用,分化成骨组织中的破骨细胞,从而导致乳腺癌相关的骨溶解[18]。
3 以MDSCs为靶点的免疫疗法大量证据表明MDSCs的累积与癌症患者临床预后密切相关。因此,临床上出现许多靶向乳腺癌MDSCs的免疫疗法。这些疗法总结起来可分为如下三种策略:抑制MDSCs的募集与增殖、清除MDSCs以及减少MDSCs的下游产物。
3.1 抑制MDSCs的募集和增殖一旦阻断MDSCs相关的细胞因子和趋化因子的产生或拮抗MDSCs表面的细胞因子受体或趋化因子受体,MDSCs的数量就会减少。使用诱导型NO合成酶(iNOS)抑制剂NG-甲基-L-精氨酸乙酸酯(NG-monomethyl-L-arginine acetate,L-NMMA)能控制MDSCs相关的骨溶解,具体机制是L-NMMA可抑制NO信号转导从而阻断MDSCs向破骨细胞的分化[18]。姜黄素(curcumin)是一种IL-6抑制剂,可以减少乳腺癌细胞中IL-6的产生,从而减少MDSCs的数量[19]。BMP4是TGF-β家族的成员,可通过阻断NF-κB途径来降低人和小鼠乳腺癌细胞中G-CSF的表达,从而减少MDSCs的分化[20]。r84是一种抗血管内皮生长因子(vascular endothelial growth factor,VEGF)抑制剂,有研究表明,其可以通过减少肿瘤内细胞因子和趋化因子的产生,特别是IL-1β,IL-6和CXCL1,来阻止MDSCs的募集[21]。血清抑制因子(serum inhibitory factors,SIF)的抑制剂sulforaphane(SFN)也可以抑制MDSCs的形成[22]。见表 1。
| 表 1 MDSCs募集/增殖抑制剂 |
通过促进MDSCs的成熟可消除MDSCs,但到目前为止,其具体机制仍尚未明确。研究表明,表达抗CD3和抗Her2/neu特异性抗体的活化T细胞(anti-Activated T cells, aATC)能够消除MDSCs,且富含Th1细胞因子(IFNγ和IL-2)的微环境可以加强aATC对MDSCs的抑制作用[23]。过继细胞疗法(adoptive cellular therapy, ACT)治疗可以减弱MDSCs的免疫抑制作用,其机制是活化的NKT细胞通过NKG2D依赖的信号通路诱导MDSCs向DCs成熟,这在小鼠和人类中已被成功验证[24]。据报道,多柔比星(doxorubicin)可通过与ROS相关的机制引发MDSCs凋亡,也可达到消除MDSCs的目的[25]。
3.3 减少MDSCs的产物降低MDSCs的产物水平对乳腺癌的治疗有积极作用。据报道,1-甲基-L-色氨酸(1-methyl-L-tryptophan, 1-MT)可抑制MDSCs产生IDO,降低MDSCs对T细胞的抑制作用,这有助于乳腺癌的治疗[26]。同样,姜黄素也可以提高LM-Mb疫苗对乳腺癌的疗效[19]。
4 小结与展望MDSCs是许多病理条件下调节免疫应答的关键因子,现在已经成为肿瘤免疫学的重要研究热点。在乳腺癌中,MDSCs不仅可以抑制抗肿瘤免疫,促进乳腺癌细胞的生长和转移,还可以降低其他免疫疗法的疗效。目前已有一些方法可以减弱MDSCs的作用,包括减少MDSCs比例和抑制其功能。此外,靶向MDSCs的免疫疗法与其他类型疗法的组合可产生协同作用。在乳腺癌MDSCs的研究中,MDSCs促进乳腺癌转移的潜在机制仍然有待深入研究。根据目前的乳腺癌分类和MDSCs有关的临床证据还不足以充分指导免疫治疗。基于MSDCs在乳腺癌中的重要作用,且其可被某些治疗方案抑制的事实,我们确信以MDSCs为靶点的免疫疗法在攻克乳腺癌的过程中具有广阔的前景。
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68: 394-424. DOI:10.3322/caac.21492 |
| [2] |
李树霞, 孔庆暖, 黄维清, 等. PES1蛋白在乳腺癌组织中的表达及临床意义[J]. 济宁医学院学报, 2018, 41(3): 180-184. DOI:10.3969/j.issn.1000-9760.2018.03.007 |
| [3] |
Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, bersion 4.2017, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Net, 2018, 16(3): 310-320. DOI:10.6004/jnccn.2018.0012 |
| [4] |
Gabrilovich DI. Myeloid-derived suppressor cells[J]. Cancer Immunol Res, 2017, 5(1): 3-8. DOI:10.1158/2326-6066.cir-16-0297 |
| [5] |
Chung W, Eum HH, Lee HO, et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer[J]. Nat Commun, 2017, 8: 15081. DOI:10.1038/ncomms15081 |
| [6] |
Valanparambil RM, Tam M, Gros PP, et al. IRF-8 regulates expansion of myeloid-derived suppressor cells and Foxp3+ regulatory T cells and modulates Th2 immune responses to gastrointestinal nematode infection[J]. PLoS Pathog, 2017, 13(10): e1006647. DOI:10.1371/journal.ppat.1006647 |
| [7] |
Chiodoni C, Sangaletti S, Colombo MP. Matricellular proteins tune myeloid-derived suppressor cell recruitment and function in breast cancer[J]. J Leukoc Biol, 2017, 102(2): 287-292. DOI:10.1189/jlb.3MR1016-447R |
| [8] |
Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment[J]. J Immunol, 2012, 189(12): 5533-5540. DOI:10.4049/jimmunol.1201161 |
| [9] |
Zou W, Bai Y, Wang X, et al. PERK-Phosphorylated eIF2alpha pathway suppresses tumor metastasis through downregulating expression of programmed death ligand 1 and CXCL5 in triple-negative breast cancer[J]. Cancer Biother Radiopharm, 2017, 32(8): 282-287. DOI:10.1089/cbr.2017.2237 |
| [10] |
Bauer D, Redmon N, Mazzio E, et al. Apigenin inhibits TNFalpha/IL-1alpha-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells[J]. PLoS One, 2017, 12(4): e0175558. DOI:10.1371/journal.pone.0175558 |
| [11] |
Tanaka T, Kajiwara T, Torigoe T, et al. Cancer-associated oxidoreductase ERO1-alpha drives the production of tumor-promoting myeloid-derived suppressor cells via oxidative protein folding[J]. J Immunol, 2015, 194(4): 2004-2010. DOI:10.4049/jimmunol.1402538 |
| [12] |
Mundy-Bosse BL, Thornton LM, Yang HC, et al. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients[J]. Cell Immunol, 2011, 270(1): 80-87. DOI:10.1016/j.cellimm.2011.04.003 |
| [13] |
Tanriover G, Eyinc MB, Aliyev E, et al. Presence of S100A8/Gr1-positive myeloid-derived suppressor cells in primary tumors and visceral organs invaded by breast carcinoma cells[J]. Clin Breast Cancer, 2018, 18(5): e1067-e1076. DOI:10.1016/j.clbc.2018.03.013 |
| [14] |
Wei L, Zhu S, Li M, et al. High indoleamine 2, 3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer[J]. Front Immunol, 2018, 9: 724. DOI:10.3389/fimmu.2018.00724 |
| [15] |
Meyer MA, Baer JM, Knolhoff BL, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance[J]. Nat Commun, 2018, 9(1): 1250. DOI:10.1038/s41467-018-03600-6 |
| [16] |
Di Cara G, Marabeti MR, Musso R, et al. New insights into the occurrence of matrix metalloproteases-2 and-9 in a cohort of breast cancer patients and proteomic correlations[J]. Cells, 2018, 7(8): 89. DOI:10.3390/cells7080089 |
| [17] |
Umansky V, Blattner C, Fleming V, et al. Myeloid-derived suppressor cells and tumor escape from immune surveillance[J]. Semin Immunopathol, 2017, 39(3): 295-305. DOI:10.1007/s00281-016-0597-6 |
| [18] |
Sawant A, Ponnazhagan S. Myeloid-derived suppressor cells as a novel target for the control of osteolytic bone disease[J]. Oncoimmunology, 2013, 2(5): e24064. DOI:10.4161/onci.24064 |
| [19] |
Liao F, Liu L, Luo E, et al. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma[J]. Arch Oral Biol, 2018, 92: 32-37. DOI:10.1016/j.archoralbio.2018.04.015 |
| [20] |
Cao Y, Slaney CY, Bidwell BN, et al. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity[J]. Cancer Res, 2014, 74(18): 5091-5102. DOI:10.1158/0008-5472.can-13-3171 |
| [21] |
Secondini C, Coquoz O, Spagnuolo L, et al. Arginase inhibition suppresses lung metastasis in the 4T1 breast cancer model independently of the immunomodulatory and anti-metastatic effects of VEGFR-2 blockade[J]. Oncoimmunology, 2017, 6(6): e1316437. DOI:10.1080/2162402x.2017.1316437 |
| [22] |
Kumar R, de Mooij T, Peterson TE, et al. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane[J]. PLoS One, 2017, 12(6): e0179012. DOI:10.1371/journal.pone.0179012 |
| [23] |
Thakur A, Schalk D, Sarkar SH, et al. A Th1 cytokine-enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid-derived suppressor cells[J]. Cancer Immunol Immunother, 2012, 61(4): 497-509. DOI:10.1007/s00262-011-1116-1 |
| [24] |
Payne KK, Zoon CK, Wan W, et al. Peripheral blood mononuclear cells of patients with breast cancer can be reprogrammed to enhance anti-HER-2/neu reactivity and overcome myeloid-derived suppressor cells[J]. Breast Cancer Res Treat, 2013, 142(1): 45-57. DOI:10.1007/s10549-013-2733-5 |
| [25] |
Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer[J]. Cancer Res, 2014, 74(1): 104-118. DOI:10.1158/0008-5472.can-13-1545 |
| [26] |
Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer[J]. J Immunol, 2013, 190(7): 3783-3797. DOI:10.4049/jimmunol.1201449 |



