2. 中国生长发育行为医学研究中心, 济宁 272029;
3. 青岛大学医学院, 青岛 266003;
4. 北京协和医院内分泌科, 北京 100730
2. Chinese Center for Behavioral Medical Research in Growth and Development, Jining 272029, China;
3. Medical College of Qingdao University, Qingdao 266003, China;
4. Department of Endocrinlolgy, Peking Union Medical College Hospital, Beijing 100730, China
生长激素缺乏症(growth hormone deficiency, GHD)是垂体前叶合成或分泌生长激素(growth hormone, GH)不足所致的全身性疾病, 可累及各年龄段人群, 临床表现多样。儿童GHD是矮小症的常见原因之一。成人GHD有肥胖、内脏脂肪增加、血脂异常及糖尿病风险增加等特征, 且其增加冠心病的风险已被公认。然而, 儿童GHD是否存在瘦体重减少、脂肪含量增加及糖脂代谢异常等心血管危险因素是目前学术界讨论的热点。目前, 与成人GHD相比儿童GHD的研究大多集中在线性生长, 对内脏脂肪的研究较少。心外膜脂肪组织被认为是内脏脂肪的标志, 测量其厚度可预测内脏脂肪的含量。目前心外膜脂肪厚度被认为是代谢性疾病及心血管疾病的新的危险因素, 在心血管疾病的发生发展中起着独立的作用[1]。本文应用超声心动图测量矮小症儿童EAT厚度, 探讨IGF-1水平与心外膜脂肪厚度的关系。
1 对象和方法 1.1 对象收集2017年1月至2017年10月在济宁医学院附属医院内分泌科住院的青春期前的矮小症患者(青春期前的标准:未进入青春发育期, Tanner分期Ⅰ期, 男孩睾丸≤4ml, 女孩乳房为B1期。矮小症标准:身高低于同种族、同性别、同年龄身高-2SD或第三百分位者), 排除合并有甲状腺疾病、遗传及代谢疾病、骨骼发育异常、慢性肝肾疾病及染色体疾病者。经知情同意, 遵循自愿原则, 共收集符合标准病例32例。每个患者均完成两个生长激素激发试验, 以生长激素激发试验峰值(GHmax)结果5ng/ml为切点, 将患者分为两组, A组:11例, GHmax<5ng/ml; B组:21例, GHmax≥5ng/ml。A、B两组患者的年龄、身高、出生体重、出生身长、父母身高、骨龄、空腹血糖(FBG)、甘油三酯(TG)、总胆固醇(TC)、低密度脂蛋白(LDL)、血尿酸(UA)无差异(P>0.05);A组体重、BMI及EAT厚度均高于B组(P<0.05), 左旋多巴生长激素激发试验峰值(L-GHmax)及胰岛素低血糖生长激素激发试验峰值(In-GHmax)均低于B组(P<0.05)。见表 1。
| 表 1 两组患者一般特征描述(x ± s) |
入选患者均收集其性别、年龄、出生身长、出生体重、目前身高、目前体重、父母身高等基本资料。其中年龄精确到月; 身高体重均在上午九点之前在空腹状态下由固定人员采用国家认证的身高测量仪(SZG-180型)测量, 数值精确到0.1cm; 体重应用电子体重秤测量, 数值精确到0.1kg。
1.2.2 化验及检查收集患者入院前1周内或者入院后完善的相关检验及检查结果, 包括肝功、肾功、甲功三项、血脂、空腹血糖、血常规、IGF-1、IGFBP3、In-GHmax及L-GHmax、骨龄、垂体核磁共振等结果。以上结果均由我院相关科室完成; IGF-1参照文献根据公式转换为IGF-1SDS[2-3]。
1.2.3 心外膜脂肪厚度应用超声心动图由同一心脏超声科医生完成。测量方法[3]:用二维超声清晰显示胸骨旁短轴切面乳头肌水平, 冻结图像于舒张末期, 在右心室前壁距室间隔2cm处垂直测量右心室前壁与心包脏层之间的无回声区作为心脏短轴切面右心室游离壁的EAT厚度, 上述参数均连续测量3个心动周期, 取其均值, 数值精确到0.1mm。
1.3 统计学方法采用易侕软件进行统计分析。定量资料用x ± s表示, 分类变量用百分数表示; 应用曲线拟合、分层分析及多因素分析观察IGF-1SDS与EAT厚度相关性。P<0.05为差异具有统计学意义。
2 结果 2.1 IGF-1及IGF-1SDS与EAT厚度相关性分析应用单因素分析发现IGF-1及IGF-1SDS与EAT无显著相关(表 2); 进一步分层分析发现, A组IGF-1与EAT仍无相关, IGF-1SDS与EAT呈负相关(β=-0.618, 95%CI:-0.954~-0.283, P=0.006), 而在B组IGF-1与IGF-1SDS均无相关性, 见表 3。
| 表 2 IGF-1SDS与EAT厚度的关系 |
| 表 3 两组IGF-1SDS与EAT厚度的关系 |
应用曲线拟合及交互作用检验发现组别对IGF-1SDS与EAT厚度相关性有交互作用(P=0.022)(见图 1, 表 4); 应用多元回归分析发现在A组IGF-1SDS与EAT厚度呈负相关(β=-0.486, 95%CI:-0.849~-0.122, P=0.015), 见表 4。
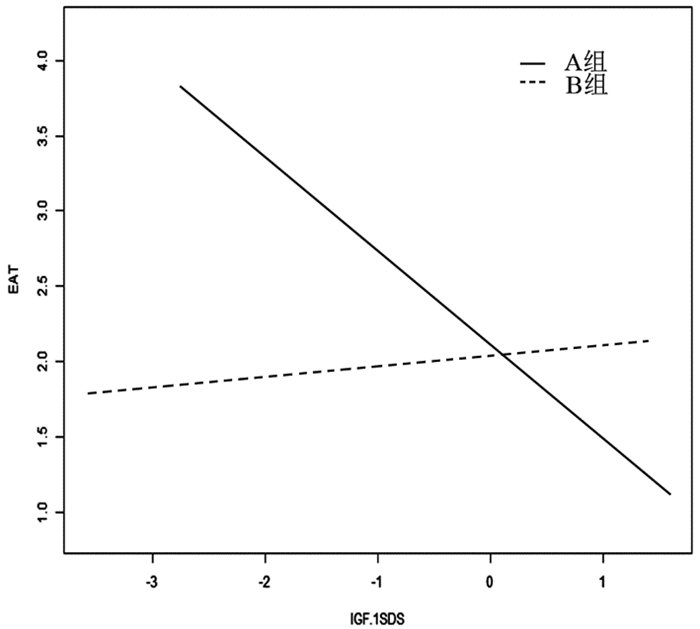
|
图 1 两组IGF-1SDS与EAT厚度关系的曲线拟合图 |
| 表 4 两组IGF-1SDS与EAT厚度关系的多元回归分析 |
GHD患者可出现脂肪堆积、血脂异常、胰岛素抵抗、炎症反应、氧化应激及凝血改变等类似代谢综合征的特征性表现, 且其心血管病的发病风险及死亡率增加。目前的结果主要来自对成人GHD研究。儿童期的生长激素缺乏是否存在瘦体重减少、脂肪量增加及对机体不利的糖脂代谢紊乱等可能会加速冠状动脉粥样硬化、增加心血管死亡率的因素一直是存在争议的问题。近年有研究表明, 粥样斑块在青春期前的儿童期即开始出现[5], 而血脂紊乱、腹部脂肪增加、胰岛素抵抗、慢性炎症、氧化应激等增加了儿童GHD患者冠心病的风险[5-6], 但目前缺少评估儿童GHD冠心病风险的有效监测指标。
心外膜脂肪组织被认为是内脏脂肪的标志, 是较其他内脏方法更具活性的代谢器官, 可产生多种炎症因子与致动脉硬化因子, 如IL-6、IL-1、TNF-α等。上述炎性介质可导致冠状动脉周围炎症和冠状动脉管壁周围平滑肌细胞增殖, 促使冠状动脉硬化狭窄。冠状动脉粥样硬化的发生可抑制具有缓解炎症、改善内皮细胞功能进而发挥抗动脉粥样硬化作用的脂联素的分泌减少, EAT厚度增加, 进而进一步加重冠脉血管的病变[7-8]。有临床观察结果显示, 近端冠脉血管因被EAT包裹的较远端更深, 致使近端的冠脉血管较远端者更易发生动脉粥样硬化[9]。同时, 有研究报道EAT厚度是冠状动脉粥样斑块易损性及冠心病危重程度的预测因子[10-11]。
本文结果显示, 生长激素完全缺乏矮小症儿童(GHmax<5ng/ml), EAT厚度较GHmax≥5ng/ml组明显增加。我们还发现, 前者BMI显著高于后者, 提示生长激素完全缺乏症儿童有体脂含量及内脏脂肪的增加, 与成人GHD病理特征相似[12]。通过分层及多因素分析发现, 生长激素完全缺乏组儿童IGF-1SDS与EAT厚度呈负相关, 调整BMI后该效应仍然存在, 证实了完全生长激素缺乏症儿童存在内脏脂肪的异常增加, 且其增加程度较体脂含量增加显著, 该效应与生长激素缺乏程度正相关, 与Khadilkar等[13]的研究结果一致。其产生机制可能与GH-IGF-1对机体的代谢影响有关。正常情况下, 机体在空腹状态时, 胰岛素分泌减少, GH-IGF-1水平升高, 促进脂解及肝糖输出; 相反, 进餐后胰岛素分泌增加, GH分泌被抑制, 从而促进骨骼肌葡萄糖摄取及糖原与脂肪的合成。而机体长期生长激素缺乏时, IGF-1长期处于低水平, 导致骨骼肌葡萄糖摄取及糖原与脂肪的合成增加; 同时IGF-1水平下降, 对前脂肪细胞的增殖、分化及凋亡的调节作用异常, 导致脂肪异常堆积[14], 且以腹部脂肪及内脏脂肪增加为主[15-16]。而EAT作为内脏脂肪的标志, 可早期反应机体组分的变化, 因此在生长激素缺乏的儿童患者EAT厚度较生长激素正常者明显增加。国外Lanes等研究亦发现GHD患儿体内的体脂含量增加, 出现了EAT堆积, 且提出这些改变是心血管疾病的重要危险因素, 与成年后心血管事件发生率及死亡率增加相关[17-18]。
IGF-1是一种较强的促有丝分裂肽, 主要在肝脏合成[19], 循环中的IGF-1水平反映了内源性的生长激素水平[2]。有研究表明, IGF-1是冠心病的独立危险因素, 且IGF-1水平与冠心病的发生风险呈负相关, 其原因可能与心肌细胞、平滑肌细胞及内皮细胞有丰富的IGF-1受体, 因此, 其对IGF-1的水平变化及其敏感有关[20]。另有研究发现, IGF-1水平越低, 心血管疾病相关的死亡率越高, 且在合并有糖脂代谢异常者更为显著[21]。流行病学研究结果也显示正常低限的IGF-1水平与急性心肌梗死、缺血性心脏病、冠状动脉粥样硬化等的发病增加有关[22]。循环中IGF-1正常低限者发生缺血性心脏病的风险是IGF-1正常高限者的1.27~1.94倍[23-24], 长期IGF-1低水平者心血管病的死亡率是一般人群的2倍。
综上所述, GHD患者心血管病变的风险增加, 且在儿童期该风险已经存在。结合EAT的易检测与观察的特点, EAT有望成为GHD患者心血管危险因素的检测指标。由于本研究目前仅通过小样本观察了矮小症儿童GHD患者生长激素治疗前基线状态下EAT厚度与生长激素正常者的差异, 下一步进行大样本队列研究, 并随访观察生长激素治疗后上述指标的变化, 以进一步验证生长激素对GHD患者身体组分及内脏脂肪等指标的影响, 为寻找简便有效评估儿童GHD冠心病风险的监测指标提供理论证据。
| [1] | Iacobellis G. Epicardial adipose tissue in endocrine and metabolic diseases[J]. Endocrine, 2014, 46(1): 8–15. DOI:10.1007/s12020-013-0099-4 |
| [2] | Wang P, Ji B, Shao Q, et al. Association between一nsulin-like growth factor-1 and Uric acid in Chinese children and adolescents with idiopathic short stature:A cross-sectional study[J]. Bio Med Research International 2018, 2018(5): 1–6. |
| [3] | Zhu H, Xu Y, Gong F, et al. Reference ranges for serum insulin-like growth factor Ⅰ(IGF-I)in healthy Chinese adults[J]. PLoS One, 2017, 12(10): e0185561. DOI:10.1371/journal.pone.0185561 |
| [4] | 张沫, 李昭屏, 李卫虹, 等. 非冠心病胸痛患者心外膜脂肪与冠状动脉血流储备的相关性[J]. 北京大学学报(医学版), 2014, 46(06): 848–853. DOI:10.3969/j.issn.1671-167X.2014.06.006 |
| [5] | De Leonibus C, De Marco S, Stevens A, et al. Growth hormone deficiency in prepubertal children:Predictive markers of cardiovascular disease[J]. Horm Res Paediatr, 2016, 85(6): 363–371. DOI:10.1159/000444143 |
| [6] | Colao A. The GH-IGF-I axis and the cardiovascular system:clinical implications[J]. Clin Endocrinol(Oxf), 2008, 69(3): 347–358. DOI:10.1111/j.1365-2265.2008.03292.x |
| [7] | engül C, zveren O. Epicardial adipose tissue:a review of physiology, pathophysiology, and clinical applications[J]. Anadolu Kardiyol Derg, 2013, 13(3): 261–265. DOI:10.5152/akd.2013.075 |
| [8] | Eiras S, Teijeira-Fernández E, Shamagian LG, et al. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue[J]. Cytokine, 2008, 43(2): 174–180. DOI:10.1016/j.cyto.2008.05.006 |
| [9] | Tellides G. Periadventitial fat[J]. Arch Pathol Lab Med, 2007, 131(3): 346–347. DOI:10.1043/1543-2165(2007)131[346:PF]2.0.CO;2 |
| [10] | Park JS, Choi SY, Zheng M, et al. Epicardial adipose tissue thickness is a predictor for plaque vulnerability in patients with significant coronary artery disease[J]. Atherosclerosis, 2013, 226(1): 134–139. DOI:10.1016/j.atherosclerosis.2012.11.001 |
| [11] | Erkan AF, Tanindi A, Kocaman SA, et al. Epicardial Adipose Tissue Thickness Is an Independent Predictor of Critical and Complex Coronary Artery Disease by Gensini and Syntax Scores[J]. Tex Heart Inst J, 2016, 43(1): 29–37. DOI:10.14503/THIJ-14-4850 |
| [12] | Jrgensen AP, Fougner KJ, Ueland T, et al. Favorable long-term effects of growth hormone replacement therapy on quality of life, bone metabolism, body composition and lipid levels in patients with adult-onset growth hormone deficiency[J]. Growth Horm IGF Res, 2011, 21(2): 69–75. DOI:10.1016/j.ghir.2011.01.001 |
| [13] | Khadilkar V, Ekbote V, Kajale N, et al. Effect of one-year growth hormone therapy on body composition and cardio-metabolic risk in Indian children with growth hormone deficiency[J]. Endocr Res, 2014, 39(2): 73–78. DOI:10.3109/07435800.2013.828742 |
| [14] | Berryman DE, Glad CA, List EO, et al. The GH/IGF-1 axis in obesity:pathophysiology and therapeutic considerations[J]. Nat Rev Endocrinol, 2013, 9(6): 346–356. DOI:10.1038/nrendo.2013.64 |
| [15] | Guha N, Nevitt SP, Francis M, et al. The Effects of recombinant human insulin-like growth factor-Ⅰ/insulin-like growth factor binding protein-3 administration on body composition and physical fitness in recreational athletes[J]. J Clin Endocrinol Metab, 2015, 100(8): 3126–3131. DOI:10.1210/jc.2015-1996 |
| [16] | Rothermel J, Lass N, Bosse C, et al. Impact of discontinuation of growth hormone treatment on lipids and weight status in adolescents[J]. J Pediatr Endocrinol Metab, 2017, 30(7): 749–757. DOI:10.1515/jpem-2017-0098 |
| [17] | Lanes R, Soros A, Flores K, et al. Endothelial function, carotid artery intima-media thickness, epicardial adipose tissue, and left ventricular mass and function in growth hormone-deficient adolescents:apparent effects of growth hormone treatment on these parameters[J]. J Clin Endocrinol Metab, 2005, 90(7): 3978–3982. DOI:10.1210/jc.2005-0091 |
| [18] | Misra M, Bredella MA, Tsai P, et al. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls[J]. Am J Physiol Endocrinol Metab, 2008, 295(2): E385–392. DOI:10.1152/ajpendo.00052.2008 |
| [19] | Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins:biological actions[J]. Endocr Rev, 1995, 16(1): 3–34. DOI:10.1210/edrv-16-1-3 |
| [20] | Strazhesko ID, Tkacheva ON, Akasheva DU, et al. Growth Hormone, Insulin-Like Growth Factor-1, Insulin Resistance, and Leukocyte Telomere Length as Determinants of Arterial Aging in Subjects Free of Cardiovascular Diseases[J]. Front Genet, 2017, 8: 198. DOI:10.3389/fgene.2017.00198 |
| [21] | Higashi Y, Sukhanov S, Anwar A, et al. Aging, atherosclerosis, and IGF-1[J]. J Gerontol A Biol Sci Med Sci, 2012, 67(6): 626–639. DOI:10.1093/gerona/gls102 |
| [22] | Colao A. The GH-IGF-I axis and the cardiovascular system:clinical implications[J]. Clin Endocrinol(Oxf), 2008, 69(3): 347–358. DOI:10.1111/j.1365-2265.2008.03292.x |
| [23] | Vasan RS, Sullivan LM, D'Agostino RB, et al. Serum insulin-like growth factor Ⅰ and risk for heart failure in elderly individuals without a previous myocardial infarction:the Framingham Heart Study[J]. Ann Intern Med, 2003, 139(8): 642–648. DOI:10.7326/0003-4819-139-8-200310210-00007 |
| [24] | Laughlin GA, Barrett-Connor E, Criqui MH, et al. The prospective association of serum insulin-like growth factor Ⅰ(IGF-I)and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults:the Rancho Bernardo Study[J]. J Clin Endocrinol Metab, 2004, 89(1): 114–120. DOI:10.1210/jc.2003-030967 |



